
Introduction
There are quite a few magnetic elements. Three - iron, cobalt and nickel - are next to each other, and are by far the most abundant ones, though some other elements, like gadolinium and dysprosium, can act like magnets too. They are ferromagnetic, which means they themselves can be magnetised, and how magnetised they are depends on the temperature of the element.
Each ferromagnetic element has a Curie point, which is the temperature at which it starts to lose its magnetic properties. If you go above the Curie point, the magnetic field exerted will gradually weaken. The elements with the highest Curie points are by far iron, cobalt and nickel, with cobalt's at 1400K. As such, they're comfortably magnetic for everyday use, such as in transformers and batteries (often as alloys, as the magnetic field is far stronger), and even in the Earth's core.
Logically, only solids should be magnetic. This however isn't exactly the case; ferrofluids are attracted to magnets, though lose any magnetic properties when a field is removed. Yet liquid magnets have been created that can maintain their magnetism, so have the ability to be made into different shapes, as this article discusses.
Why are only some elements magnetic?
The reason why the aforementioned trio can is due to the spin of their electrons aligning:
- All electrons have spin of +1/2 or -1/2. These electrons will each have a magnetic moment which comes about from an electron's motion and spin.
- Electrons are arranged in sub-orbitals, with each orbital containing two electrons of opposite spin. This would mean the magnetic moments cancel each other out, so the atom has no net magnetic field.
- However, in the case of the aforementioned trio, the magnetic moments don't cancel out; they permanently align in parallel with each other. These moments contribute to a net magnetic field forming.
- Iron, cobalt and nickel have the most unpaired electrons per atom, so have the strongest magnetic fields. However, compounds with other transition metals, like chromium, can be magnetic too.
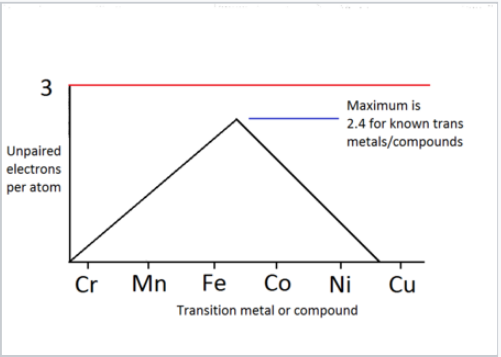
This diagram is courtesy of LibreTexts, at this link. From here it's hopefully clearer that compounds, like Cr2O3 and MnO2, can be magnetic (though less than iron compounds) but copper compounds won't be, due to the number of unpaired electrons.
Overall, we can expand this definition:
- If there are unpaired electrons, there will be a magnetic field, though weaker to that of iron. These atoms would be paramagnetic, and will not be permanently attracted to a magnet.
- If the electrons are all paired up, there will be no overall magnetic field. These atoms are diamagnetic, and will repel a magnet.
There are other forms of magnetism as well:
- Antiferromagnetism is where the magnetic moments align anti-parallel to each other.
- Essentially, magnetic moments are vectors which point in a certain direction. In ferromagnets, the vectors point in the same direction. In antiferromagnets, the vectors point either north or south.
- Antiferromagnets are present below a certain temperature - the Neel point - though above this temperature, they are paramagnetic. They're quite rare and are a relatively new discovery - Louis Neel won the Nobel Prize in Physics for discovering them in 1970.
- Ferrimagnetism is where the moments are anti-parallel and of different magnitudes below the Curie point. They're thus both like antiferromagnets and ferromagnets.
Here's a basic diagram showing the differences in the orientation of magnetic moments in ferromagnets and antiferromagnets. This occurs in antiferromagnets due to ions spontaneously shifting into opposite arrangements at low temperatures, a process which is similar to how ferrimagnets come about.
We can also define these types of magnetism by how susceptible they are at being magnetised - their magnetic suspectibility, or χ.; the greater it is, the more easily a material will be magnetised. Notably, χ is inversely proportional to temperature, which mathematically explains why, as the temperature approaches the Curie point, a material's magnetic field gets weaker. As already mentioned, this is luckily not an issue at room temperature for many metals.
Separating with magnets
There are many uses for magnets, such as in landfill to find valuable metals. Upon searching for landfill magnets, I found that magnets aren't typically recycled since they can be hazardous to the environment. You'd have to demagnetise it, which can be difficult if it's ferromagnetic, yet there seems to be a unique solution to the issue: use hydrogen.
They're building a rare earth magnet recycling plant in Birmingham, which uses Hydrogen Processing of Magnetic Scrap (HPMS) that can produce new magnets as well as demagnetising old ones, whilst seemingly being cleaner than other processes. An article from the University of Birmingham on the recycling plant can be found here.
Magnets are also useful in catalysis; iron and nickel are used as catalysts in reactions not for their magnetic properties as well. You can separate a catalyst from a reaction mixture using magnets, as this paper discusses. Superparamagnetic nanoparticles act like paramagnets in a magnetic field, but once it is removed, they have no magnetic properties. Using them in separation means catalysts can be removed and recycled more easily, with it also being cleaner than other separation methods like filtration. The paper goes far more in depth about it, though.
 |
| Iron filing in glycerol |
Epilogue
I could carry on discussing magnets, though by that point I'd be veering further into the physics side of how they work - that would deserve its own blogpost. However, I think I've finally managed to figure out a question I've wanted an answer to for a few years - how do magnets work?
Image of gadolinium from Wikipedia: https://en.wikipedia.org/wiki/File:Gadolinium-4.jpg
Mentions of "magnet"-like words in this blogpost: 72

Comments
Post a Comment