This was supposed to be an add-on to the carbon blogpost, but took on a life of its own. I regret my life choices /s.
Here's an extremely boring chemistry question:
Can you have a C2 molecule? If so, what would its structure look like?
You might think this is what it would look like:
 |
| Our proposed dicarbon molecule |
So does it exist? Maybe.
You see, there's a gas called dicarbon, which is similar to the molecule I've drawn above, except instead of a quadruple bond between the carbons, there's a double bond, and each carbon has a lone pair as well. We can deduce that there's a double bond due to molecular orbital theory, which states the bond order of dicarbon ought to be 2. I'd draw out a diagram below, but I studied MO theory last term at uni and it traumatised me a bit.
 |
| What everyone agrees dicarbon is like |
But I happened to find two articles from 2012 - one from Chemistry World, another from Nature - discussing research at the Hebrew University of Jerusalem - which claim this quadruple carbon bond is possible in dicarbon. Put simply, they say it's due to quantum mechanics, where a regular triple bond exists between the carbons, but an additional fourth bond is achieved by two electrons in hybrid orbitals pointing away from the central bonding region.
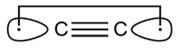 |
| Possible quadruple bond. Source: Chemistry World article linked above |
Lead researcher Sason Shaik even said ’For many chemists, this looks impossible'. And, yeah, I agree.
The real strange thing is that quadruple bonds do exist, they're merely most common amongst transition metals because of their d orbitals. They end up forming delta bonds! Although I'm not sure if this applies to our hypothetical dicarbon, because if it does bond as Shaik's research indicates, we don't end up with d orbitals overlapping each other as you'd get in, say, this complex below. Because carbon doesn't have any convenient d orbitals.
 |
| Potassium octachlorodirhenate |
Delta bonds were first theorised by Robert Mulliken in 1931, and in 1964, this compound was the first to be discovered as having such bonds. Specifically, let's focus on the octachlorodirhenate ion [Re2Cl8]2-, which already looks ridiculous, not least as that quadruple bond is actually very stable.
 |
| The colour of the [Re2Cl8]2- ion. Source: https://en.wikipedia.org/wiki/File:(NBu4)2Re2Cl8.jpg |
In fact, if you were to look at this compound, you'd notice it's a blue-green powder. This is because of δ to δ* electronic transitions, which is energetically equivalent to a wavelength in that part of the visible light spectrum.
But the first quadruple bond involving molecule to be synthesised was back in 1844, though at the time, no one realised it. It also has a far more typical name: chromium (II) acetate [Cr2(CH3CO2)4(H2O)2].
 |
| chromium (II) acetate |
The structure isn't that weird, either - two chromiums, four acetate ions, two water molecules, with them all acting as ligands. But look at that quadruple bond! A similar compound exists, this time with a rhenium (II) metal ion, in fact.
The maximum bond order detected is 6, as in sextuple bonds. There are only two molecules like this, though, in dimolybdenum (Mo2) and ditungsten (W2). Where both Mo and W are metals at room temperature and pressure, these diatomic counterparts exist in the gaseous phase - for example, exciting a molybdenum sheet at 7K will produce Mo2 - which I find so ridiculous. In fact, Mo2 and W2 end up being outliers, because once you even manage to get close to sextuple bonds, the d electrons tend to ferromagnetically couple instead of bonding covalently. And if you're wondering what bond types are present - it's two σ, π, and δ bonds each.
 |
| Ditungsten, in its sextuple bond glory. ChemDraw doesn't even allow this many bonds! |
And according to this paper, sextuple bonds are the limit because once you reach the f block, the f orbitals contract too close to the nucleus to be involved in bonding anyways.
So no, dicarbon probably doesn't have quadruple bonds. It might, but it seems unlikely. But those kinds of bonds do exist, and you can even go higher than that.
Extra difficult😩
ReplyDeleteSextuple bonds? 😵💫
ReplyDelete